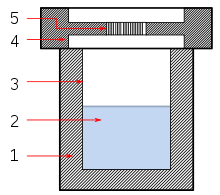
용열 합성
Solvothermal synthesis솔보온 합성은 화학 화합물을 생산하는 방법이다.그것은 열수 경로와 매우 유사하다.둘 다 일반적으로 스테인리스강 고압 멸균기에서 전도됩니다.유일한 차이점은 전구체 용액이 보통 비수성이라는 것이다.대표적인 용제로는 디메틸포름아미드와 다양한 [1]알코올이 있습니다.
MOF,[2][3] [4]이산화티타늄, 그래핀,[5] 탄소구,[6] 칼코게나이드[7] 및 기타 물질들을 만들기 위해 솔보온 합성이 사용되어 왔다.
레퍼런스
- ^ Demazeau, G. (2008). "Solvothermal reactions: an original route for the synthesis of novel materials". J. Mater. Sci. 43 (7): 2104–2114. Bibcode:2008JMatS..43.2104D. doi:10.1007/s10853-007-2024-9. S2CID 55825984.
- ^ Yaghi, O. M.; Kalmutzki, M. J.; Diercks, C. S. (2019). Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks. Weinheim: Wiley-VCH. ISBN 978-3-527-82110-5.
- ^ Farha, Omar K.; Hupp, Joseph T. (2010). "Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials". Accounts of Chemical Research. 43 (8): 1166–1175. doi:10.1021/ar1000617. PMID 20608672.
- ^ Ong, Wee-Jun; Tan, Lling-Lling; Chai, Siang-Piao; Yong, Siek-Ting; Mohamed, Abdul Rahman (2014). "Highly reactive {001} facets of TiO2-based composites: Synthesis, formation mechanism and characterization". Nanoscale. 6 (4): 1946–2008. Bibcode:2014Nanos...6.1946O. doi:10.1039/c3nr04655a. PMID 24384624.
- ^ Xiang, Quanjun; Yu, Jiaguo; Jaroniec, Mietek (2012). "Graphene-based semiconductor photocatalysts". Chem. Soc. Rev. 41 (2): 782–796. doi:10.1039/C1CS15172J. PMID 21853184.
- ^ Hu, Gang; Ma, Ding; Cheng, Mojie; Liu, Lin; Bao, Xinhe (2002). "Direct synthesis of uniform hollow carbon spheres by a self-assembly template approach". Chemical Communications (17): 1948–1949. doi:10.1039/B205723A. PMID 12271688.
- ^ Li, J.; Chen, Z.; Wang, R. J.; Proserpio, D. M. (1999). "Low temperature route towards new materials: Solvothermal synthesis of metal chalcogenides in ethylenediamine". Coordination Chemistry Reviews. 190–192: 707–735. doi:10.1016/S0010-8545(99)00107-1.