Air displacement pipette
Piston-driven air displacement pipettes are a type of micropipette, which are tools to handle volumes of liquid in the microliter scale. They are more commonly used in biology and biochemistry, and less commonly in chemistry; the equipment is susceptible to damage from many organic solvents.
Operation
These pipettes operate by piston-driven air displacement. A vacuum is generated by the vertical travel of a metal or ceramic piston within an airtight sleeve. As the piston moves upward, driven by the depression of the plunger, a vacuum is created in the space left vacant by the piston. Air from the tip rises to fill the space left vacant, and the tip air is then replaced by the liquid, which is drawn up into the tip and thus available for transport and dispensing elsewhere.
Sterile technique prevents liquid from coming into contact with the pipette itself. Instead, the liquid is drawn into and dispensed from a disposable pipette tip that is changed between transfers. Depressing the tip ejector button removes the tip, that is cast off without being handled by the operator and disposed of safely in an appropriate container. This also prevents contamination of or damage to the calibrated measurement mechanism by the substances being measured.
플런저는 액체를 끌어 올리고 분사할 수 있도록 눌려 있다. 정상 작동은 피펫이 공기 중에 있는 동안 플런저 버튼을 첫 번째 정지 지점까지 누르는 것으로 구성된다. 그런 다음 팁은 운반할 액체에 잠기고 플런저는 느리고 고른 방식으로 방출된다. 이렇게 하면 액체가 끝부분으로 빨려 들어간다. 그런 다음 기기는 원하는 분사 위치로 이동한다. 플런저는 다시 첫 번째 정지 지점까지 눌린 다음 두 번째 정지 지점, 즉 '폭발' 위치로 이동한다. 이 동작은 팁을 완전히 제거하고 액체를 분사한다. 조절 가능한 피펫에서, 팁에 포함된 액체의 부피는 가변적이다; 모델에 따라 다이얼이나 다른 메커니즘을 통해 바뀔 수 있다. 일부 피펫은 현재 선택된 볼륨을 표시하는 작은 창을 포함한다. 플라스틱 피펫 팁은 수용액용으로 설계되었으며, 팁 또는 피펫의 플라스틱을 용해시킬 수 있는 유기 용제와 함께 사용하지 않는 것이 좋다.
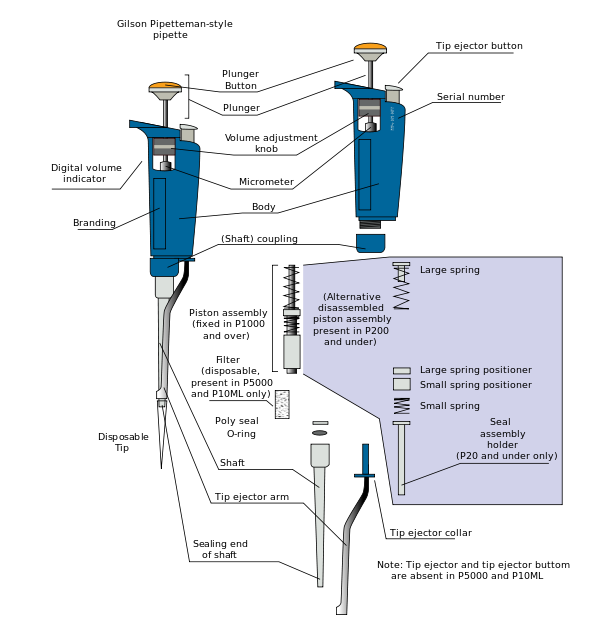
- 마이크로피펫의[1] 주요 부품
- 플런저 버튼
- 팁 배출기 버튼
- 볼륨 조절 다이얼
- 디지털 볼륨 표시기
- 축
- 일회용 팁의 부착 지점
모델
다음과 같은 몇 가지 다른 유형의 공기 변위 피펫이 존재한다.
- 조정 가능하거나 고정된
- 취급량
- 단일 채널 또는 멀티 채널 또는 리피터
- 조절 가능한 팁 간격
- 원뿔형 끝 또는 원통형 끝
- 표준 또는 잠금
- 수동 또는 전자적
- 제조자
조절 가능 또는 고정 볼륨
마이크로피펫은 최소 0.2 µL, 최대 10,000 µL(10mL)의 볼륨을 사용할 수 있다.[2][3] 따라서 그것들은 5, 10, 25, 50 mL 부피에 달하는 졸업된 피펫과 같은 장비보다 더 작은 규모의 전송에 사용된다.
가장 일반적인 유형의 피펫은 그 작동 범위 내에서 특정 볼륨으로 설정될 수 있으며, 조절 가능한 피펫이라고 불린다. 이러한 피펫에는 일반적으로 "10–100 µL"과 같은 볼륨 범위가 있는 라벨이 있다. 이러한 한도를 덮어쓰면 피펫팅 시스템이 손상되기 때문에 이러한 한계는 실제로 한계치인 것이다. 고정 볼륨 피펫은 변경할 수 없다. 움직이는 부품이 적기 때문에 메커니즘이 덜 복잡해 더욱 정확한 볼륨 측정이 이뤄진다.
In 1972, several people of the University of Wisconsin–Madison (mainly Warren Gilson and Henry Lardy) enhanced the fixed-volume pipette, developing the pipette with a variable volume.[4] Warren Gilson founded Gilson Inc. based on this invention.
Volume
This section needs expansion. You can help by adding to it. (December 2009) |
For optimal usage, every pipette supplier offers a broad range of different capacities. A small volume range of a pipette like 10–100 µL results in a much higher accuracy than a broad range from 0.1–1,000 µL per pipette.
With regard to the volume transferred, the smallest pipette that can handle the required volume should be selected. This is important because accuracy decreases when the set volume is close to the pipette’s minimum capacity. For example, if 50 µl are dispensed using a 5,000 µl pipette, the results will be rather poor. Using a 300 µl pipette will give better results, whereas using a 50 µl pipette would be ideal.[5]
Tips
For the pipetting process there are two components necessary: The pipette and disposable tips. The tips are plastic-made tools for single-use. In general, they are made of Polypropylene. Depending on the size of the pipette, the user needs specific tip sizes like: 10 µL, 100 µL, 200 µL, 1,000 µL, other non-standard sizes, such as 5,000 µL (5 mL) or 10,000 µL (10 mL). The majority of tips have a color code for easy spotting like natural (colorless) for low volumes (0.1–10 µL), yellow (10–100 µL), or blue (100–1,000 µL). The corresponding pipette has the same color code, printed on the pipette.
For special applications, there are filter-tips available. These tips have a little piece of foam plastic in the upper conus to prevent sample aerosols contaminating the pipette.
In general, all tips are stored in 8 × 12 boxes for 96 pieces in an upright position. The spacing of tips in these boxes is usually standardised for multichannel pipette compatibility from a number of different suppliers.
| Name | Min. volume (µL) | Max. volume (µL) | Color on Gilson[clarification needed] | tip size (µL) | |
|---|---|---|---|---|---|
| P2 | 0.2 | 2 | Orange | 10 | |
| P10 | 1 | 10 | Red | 10 | |
| P20 | 2 | 20 | Lemon | 200 | |
| P100 | 20 | 100 | Salmon | 200 | |
| P200 | 50 | 200 | Yellow | 200 | |
| P1000 | 200 | 1000 | Blue | 1000 | |
| P5000 | 500/1000 | 5000 | Purple | 5000 | |
| P10000 | 1000 | 10000 | Sky | 10000 | |
Two major tip systems exist, called conical or cylindrical, depending on the shape of the contact point of the pipettes and the tip.[6]
Single-channel and multi-channel pipettes
Depending on the number of pistons in a pipette, there is a differentiation between single-channel pipettes and multi-channel pipettes. For manual high-throughput applications like filling up a 96-well microtiter plate most researchers prefer a multi-channel pipette. Instead of handling well by well, a row of 8 wells can be handled in parallel as this type of pipette has 8 pistons in parallel.
Adjustable tip spacing pipettes
Some manufacturers offer adjustable tip spacing pipettes. These allow to transfer multiple samples in parallel between different labware formats.
Electronic pipettes
To improve the ergonomics of pipettes by reducing the necessary force, electronic pipettes were developed. The manual movement of the piston is replaced by a small electric motor powered by a battery. Whereas manual pipettes need a movement of the thumb (up to 3 cm), electronic pipettes have a main button. The programming of the pipette is generally done by a control wheel and some further buttons. All settings are displayed on a small display. Electronic pipettes can decrease the risk of RSI-type injuries.[citation needed]
Repeaters
Repeaters are specialized pipettes, optimized for repeated working steps like dispensing several times a specific volume like 20 µL from a single aspiration of a larger volume. In general, they have specific tips which do not fit on normal pipettes. Some electronic pipettes are able to perform this function using standard tips.
Locking mechanism
Some air displacement pipettes can additionally feature a locking mechanism (referred to as "locking pipettes") to allow better changing of volume yet preserving accuracy. By locking the set volume while performing several identical pipetting actions, accidental changes to the pipette volume setting are avoided. The lock mechanism is typically a mechanical toggle close to the pipette setting controls that interferes with the setting mechanism to prevent movement. Some pipettes, however, feature dials for setting the individual volume digits that can only be adjusted when unlocked by depressing and twisting the plunger.[7]
Calibration
For sustained accuracy and consistent and repeatable operation, pipettes should be calibrated at periodic intervals. These intervals vary depending on several factors:
- The skill and training of the operators. Skilled operators tend to operate the instrument more correctly and make fewer accuracy-robbing mistakes.
- The liquid dispensed by the pipette. Corrosive and volatile liquids tend to emit vapors which ascend into the pipette shaft even under proper operating conditions and may corrode the metal piston and springs, or the seals and o-rings that provide an air-tight seal between the piston and the surrounding sleeve.
- Proper and careful handling. Pipettes that are frequently dropped, are subjected to careless handling or horseplay, or that are not properly stored in a vertical position, will tend to degrade in accuracy over time.
- The accuracy required by the instrument. Applications requiring maximum accuracy also demand more frequent calibration. Instruments used for purely research applications or in educational settings generally require less frequent calibration.
Under average conditions, most pipettes can be calibrated semi-annually (every six months) and provide satisfactory performance. Institutions that are regulated by the Food and Drug Administration's GMP/GLP regulations generally benefit from quarterly calibration, or every three months. Critical applications may require monthly service, while research and educational institutions may need only annual service. These are general guidelines and any decision on the appropriate calibration interval should be made carefully and include considerations of the pipette in question (some are more reliable than others), the conditions under which the pipette is used, and the operators who use it.
Calibration is generally accomplished through means of gravimetric analysis. This entails dispensing samples of distilled water into a receiving vessel perched atop a precision analytical balance. The density of water is a well-known constant, and thus the mass of the dispensed sample provides an accurate indication of the volume dispensed. Relative humidity, ambient temperature, and barometric pressure are factors in the accuracy of the measurement, and are usually combined in a complex formula and computed as the Z-factor. This Z-factor is then used to modify the raw mass data output of the balance and provide an adjusted and more accurate measurement.
The colormetric method uses precise concentrations of colored water to affect the measurement and determine the volume dispensed. A spectrophotometer is used to measure the color difference before and after aspiration of the sample, providing a very accurate reading. This method is more expensive than the more common gravimetric method, given the cost of the colored reagents, and is recommended when optimal accuracy is required. It is also recommended for extremely low-volume pipette calibration, in the 2 microliter range, because the inherent uncertainties of the gravimetric method, performed with standard laboratory balances, becomes excessive. Properly calibrated microbalances, capable of reading in the range of micrograms (10−6 g) can also be used effectively for gravimetric analysis of low-volume micropipettes, but only if environmental conditions are under strict control. Six-place balances and environmental controls dramatically increase the cost of such calibrations.
Additional images
References
- ^ "Use of Micropipettes" (PDF). buffalostate.edu. Retrieved 19 June 2016.
- ^ "Volumetric Measurement in the Laboratory" (PDF). brand.de. Retrieved 6 July 2016.
- ^ Henry, Kelli. "How to Use a Micropipette" (PDF). mcdb.ucla.edu. Retrieved 19 June 2016.
- ^ Zinnen, Tom (June 2004). "The Micropipette Story". The Board of Regents of the University of Wisconsin System. Archived from the original on 26 December 2009. Retrieved 14 December 2009.
- ^ "Are you using the right type of micropipette? [How-to]". INTEGRA Biosciences. Retrieved 20 August 2020.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 30 November 2010. Retrieved 15 September 2009.CS1 maint: archived copy as title (link)
- ^ "EVOLVE Well Balanced Manual Pipettes". INTEGRA Biosciences.








